Needles are critical components of a syringe for injecting medications into the body or withdrawing fluids from it. This application focuses on needle inspection after they’re formed and before they’re assembled with the hub and plunger into the final syringe.

What manufacturing defects occur?
Defects can occur during the needle formation process due to a variety of factors such as material quality, equipment and tool maintenance, process control, and human error. Common defects include:
- Bent Needles– Bent needles that make injections difficult or impossible
- Irregular Needle Sharpness– A poorly formed or blunt tip which can cause painful injections or damage during puncture
- Surface Imperfections– Needle surface imperfections such as scratches and pits that can cause discomfort during injection
- Burrs– Tiny fragments of metal that form at the edges of needles, especially after grinding, that can cause pain or tissue damage during injection
- Incorrection Dimensions– Variations in length or diameter of needles due to errors in cutting or forming processes, which can affect dosage accuracy and compatibility with the syringe hub
These defects can compromise the functionality, effectiveness, and comfort of an injection, ultimately putting patient safety at risk. Quality escapes can lead to costly recalls, legal issues, and damaged reputation with healthcare providers and patients. It is crucial for manufacturers to rigorously inspect needles to prevent escapes. And by inspecting needles after formation and identifying issues early, manufacturers reduce waste and improve efficiency.
However, these defects can be difficult to detect– needle surfaces, made of steel, may reflect light in a way that masks defects. And many needle formation defects are subtle, making them difficult to detect, especially if the defect’s contrast to the background is low. Traditional machine vision systems struggle to capture clear images and distinguish between actual defects, reflective surfaces, and the background, ultimately missing defects or causing false rejections.
And in high-volume manufacturing environments, needles must be inspected quickly to keep up with production rates. Traditional machine vision products may fail to keep up with required cycle times.
The Solution
UnitX’s AI-powered inspection effectively detects syringe needle formation defects where other solutions fail.
First, the OptiX imaging system illuminates and images needles. Then, the CorteX AI platform is trained on needle formation defects. Lastly, those AI models are deployed to the CorteX inference system to detect and classify defects in-line.
Why UnitX for syringe needle formation inspection?
OptiX provides superior images that minimize reflectivity while maximizing defect visibility. It has 32 independently controllable lighting sources that can be optimized for needles’ steel surfaces and various defects via software. Its computational imaging capability can be used to take multiple shots and eliminate hotspots caused by highly reflective needle surfaces. And its lighting dome design supports a very acute incidence angle of projected light, causing even very tiny defects to cast shadows which increase their visibility.
CorteX accurately detects random, complex defects. It automatically normalizes for variability in positions and orientations and recognizes defects down to the pixel-level. It reduces false positives that lead to scrap and wasted product.
CorteX supports fast AI model development, deployment, and iteration. CorteX AI models are sample efficient– they only require a few images to train on new defect types.
UnitX optimizes yield. In CorteX, can tune quality criteria and visualize the impact on yield before rolling those changes to production. All inspection data is referenceable in one central platform for manufacturers to analyze and identify areas for process improvements.
UnitX provides rapid, 100% inline inspection. OptiX has bright LEDs and fast fly capture speeds of 1m/s for high speed imaging. And CorteX supports high inference speeds (up to 100 MP) to quickly output an OK/NG decision, seamlessly communicating that decision via integration to all major PLC, MES, and FTP systems.
Manufacturers who use UnitX to automate needle inspection are able to:
- Prevent quality escapes that put patient safety and comfort at risk
- Reduce scrap by identifying defects early in the manufacturing process and minimizing false rejection rates common with traditional machine vision
- Improve yield by analyzing production and quality data for process improvement opportunities
- Automate inspection at the speed of their production to increase manufacturing throughput
UnitX Inspection Example Deep Dive
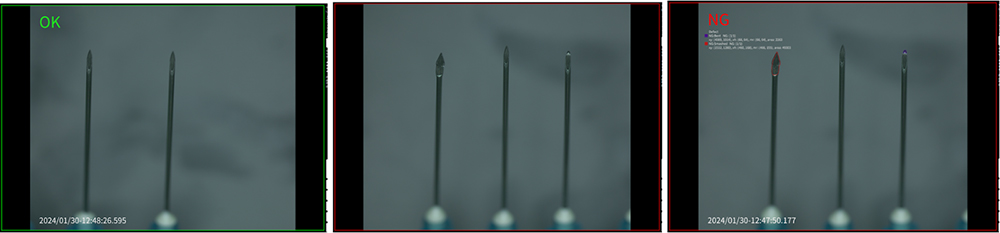
In this example, we inspected needles after they’re formed and before they’re assembled with syringe components to catch defects early and minimize waste. We specifically looked for bent needle tips.
Imaging
First, we used OptiX to capture images of the needles, making sure we captured both defective and OK parts.
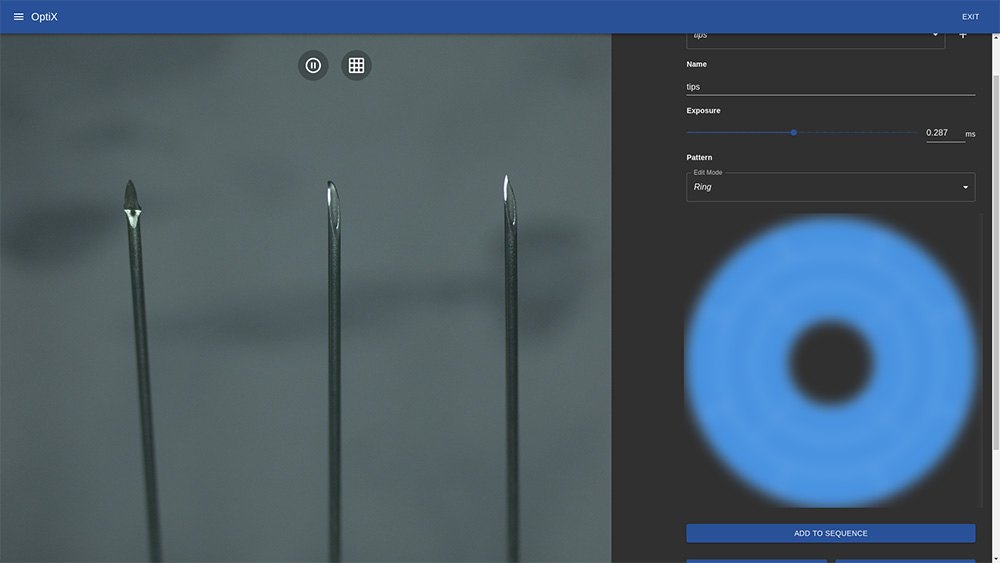
Training
Next, we used CorteX to train our models. We created labels to represent the bent needle defect. We then labeled those defects in the images we captured from OptiX, using only 4 images of NG parts.
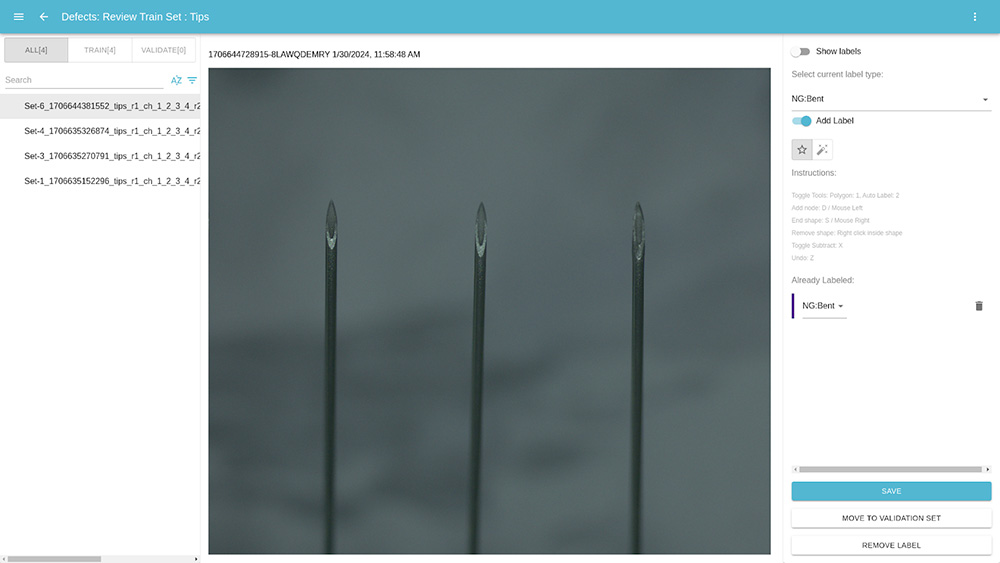
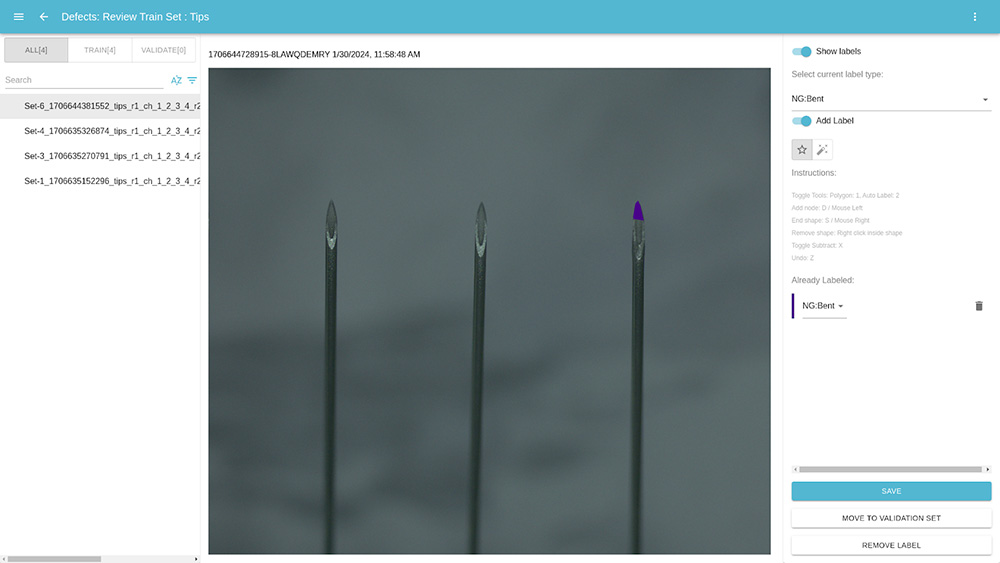
Because of CorteX’s user friendly interface and the low number of images it requires to train its AI models, it only took us a few minutes to label the defects in the images.
Detection
We then deployed those AI models to CorteX to detect defects on new needle parts, resulting in the accurate detection and classification of bent needle defects.